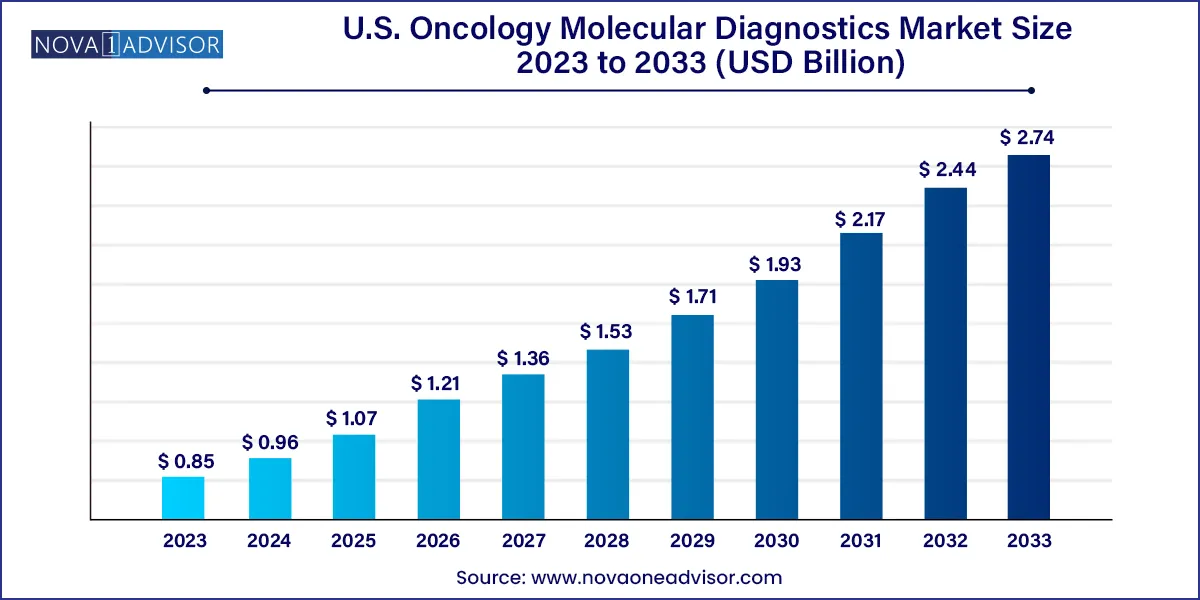
The U.S. oncology molecular diagnostics market size was estimated at USD 0.85 billion in 2023 and is projected to hit around USD 2.74 billion by 2033, growing at a CAGR of 12.41% during the forecast period from 2024 to 2033.
U.S. Oncology Molecular Diagnostics Market Key Takeaways
- By type, the breast cancer segment dominated the market in 2023.
- By type, the liver cancer segment is expected to grow at a notable rate in the market during the forecast period.
- By technology, the polymerase chain reaction (PCR) segment held the largest market share in 2023.
- By technology, the sequencing segment is expected to experience significant growth in the market during the forecast period.
- By product, the reagents segment is dominated the market in 2023.
- By product, the instruments segment is expected to grow at a considerable rate during the forecast period.
U.S. Oncology Molecular Diagnostics Market Overview
Oncology molecular diagnostics is used to detect proteins, materials, genetics, or molecules that contain information about cancer in the body. The increased global prevalence of cancer is driving the introduction of oncology molecular diagnostic tools for identifying and studying cancer cells' biochemical characteristics. The market's growth is accelerated by increased awareness of cancer therapy and diagnosis, as well as technological developments in the diagnostic procedure. The rising occurrence of cancer in the United States as a result of changing lifestyles and increased sedentary behavior is driving the expansion of the US oncology molecular diagnostics market.
U.S. Oncology Molecular Diagnostics Market Report Scope
| Report Attribute |
Details |
| Market Size in 2024 |
USD 0.96 Billion |
| Market Size by 2033 |
USD 2.74 Billion |
| Growth Rate From 2024 to 2033 |
CAGR of 12.41% |
| Base Year |
2023 |
| Forecast Period |
2024 to 2033 |
| Segments Covered |
By Type, By Technology, and Product |
| Market Analysis (Terms Used) |
Value (US$ Million/Billion) or (Volume/Units) |
| Report Coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
| Key Companies Profiled |
Roche Diagnostics, Abbott Laboratories, Thermo Fisher Scientific Inc., Illumina, Inc., Bio-Rad Laboratories, Inc., Myriad Genetics, Inc., Agilent Technologies, Inc., Danaher Corporation (Cepheid), Hologic, Inc., Foundation Medicine, Inc. and Others. |
U.S. Oncology Molecular Diagnostics Market Growth
The growth of the U.S. oncology molecular diagnostics market is fueled by several key factors. Firstly, advancements in technology have led to the development of more precise and efficient molecular diagnostic tests, enabling earlier detection and personalized treatment of cancer. Additionally, the increasing prevalence of cancer cases in the United States has driven the demand for diagnostic tools that can accurately identify specific biomarkers associated with different types of cancer. Moreover, the rising adoption of targeted therapies and immunotherapies, which rely on molecular diagnostic testing for patient stratification and monitoring treatment response, has further propelled market growth. Furthermore, supportive government initiatives and investments in cancer research and healthcare infrastructure have contributed to the expansion of the molecular diagnostics market in oncology, ensuring access to innovative diagnostic solutions for patients across the country.
U.S. Oncology Molecular Diagnostics Market Dynamics
Driver: the rising advancements in the diagnostics process
The increasing adoption of molecular diagnostics in the treatment and the detection of the cancer that driving the growth of the market. Molecular diagnostic is used for selecting the therapy or treatment based on biomarkers identified from the Transcriptome (mRNA), and tumor's genome (DNA). Molecular diagnostics helps to analyze clear information about the molecular condition of tumor which helps in improvement in the treatment of the patients. Precision medicine helps in treating the patients and helps in improving the quality-of-life patient by understanding the biological condition of their patients. The increasing investments in research and development on molecular diagnostics helps to enhance technologies for the molecular picture of cancer. Thus, the advancements and increasing investments in the diagnostics process that boosts the growth of the U.S. oncology molecular diagnostics market.
Restraint: high cost of diagnostics
The estimated high cost of diagnostic processes for cancer in the United States is observed to hamper the growth of the U.S. oncology molecular diagnostics market. Developing and commercializing new molecular diagnostic tests for cancer involves substantial research and development costs, regulatory requirements, and market access considerations. The high upfront investment and uncertainty surrounding market acceptance may discourage companies from investing in the development of new diagnostic technologies. Some molecular diagnostic tests for cancer may require specialized equipment, expertise, and facilities that are not readily available in all healthcare settings. Patients living in rural or underserved areas may face challenges accessing these specialized testing centers, leading to disparities in diagnostic testing and treatment outcomes.
Opportunity: rising emphasis on precision solutions
The development in cancer medication as a shift in the personalized or precision solutions for better results and treatment outcomes is observed to expand the overall molecular diagnostics market in the United States. Precision oncology emphasizes the customization of cancer treatment plans based on the individual molecular characteristics of a patient's tumor. Molecular diagnostics play a crucial role in identifying specific genetic mutations, biomarkers, and molecular signatures associated with cancer subtypes, drug responses, and disease progression. As the demand for personalized medicine continues to grow, there is increasing reliance on molecular diagnostics to guide treatment decisions and optimize patient outcomes.
U.S. Oncology Molecular Diagnostics Market By Type Insights
The breast cancer segment dominated the U.S. oncology molecular diagnostics market with the largest share in 2023. The rising prevalence of breast cancer due to the damage, mutation, and DNA in breast cells. Cancer cells start to grow in the milk producing lobules of the breast that further causes breast cancer, especially in females. Molecular diagnostics play a crucial role in breast cancer screening and early detection. Tests such as BRCA gene mutation testing and HER2/neu testing help identify individuals at increased risk of developing breast cancer or those with specific molecular subtypes of the disease. Early detection enables timely intervention and improves patient outcomes.
- The breast cancer is majorly impacted females with approximately 99% and 0.5-1% in the males. The increasing integration of the next-generation sequencing technologies for improving the genetic profiling drives more effective diagnosis for breast cancer.
The liver cancer segment is expected to grow at a notable rate in the U.S. oncology molecular diagnostics market during the forecast period. The liver cancer is the fastest growing type of cancer in the United States. Cancer in liver, and cancer in bile ducts of liver are the two major types of liver cancer. The rising the acceptance of the sedentary lifestyle is causing the higher number of liver cancer in the U.S. population. Molecular diagnostics helps in the early detection and the treatment of the diseases that boosts the adoption of the market.
U.S. Oncology Molecular Diagnostics Market By Technology Insights
The polymerase chain reaction (PCR) segment held the largest share in the U.S. oncology molecular diagnostics market in 2023. The growth of the segment is attributed to the rising adoption of the polymerase chain reaction (PCR) into the diagnosis method for the cancer for better and accurate results. Polymerase chain reaction (PCR) is one of the advancements in the technologies for the detection of several cancers. The technological evolution in the polymerase chain reaction (PCR) are digital PCR, and quantitative PCR (qPCR) that improves the quality of precision and accuracy in the detection of cancer specific genetics.
The sequencing segment is expected to experience a significant growth in the U.S. oncology molecular diagnostics market during the forecast period. Next-generation sequencing is one of the important methods for detecting and diagnostics of cancer and redefining cancer treatment from past several years. The rising investments in the research and development activities in sequencing technologies for the improvement and accuracy in the cancer related genetics alteration promotes the segment’s growth. The advancements in sequencing technologies are driving the growth in the adoption of oncology molecular diagnostics.
U.S. Oncology Molecular Diagnostics Market By Product Insights
The reagents segment is dominated the market in 2023. The growth of the segment is attributed to the rising adoption of reagents for the diagnostics and testing in cancer research. The rising development in biotechnology, molecular biology technology, and synthetic biology that driving the demand for the segment. Additionally, the rising investments in research and development activities by the biotech companies for the development of the diagnostics process further boosts the growth of the segment in the market.
The instruments segment is expected to increase its U.S. oncology molecular diagnostics market growth during the predicted period. The rising demand for the diagnostics specialized instruments and devices for the genetic and molecular analysis that boosting the demand for the instruments segment. The diagnostics instruments include PCR machines, and DNA sequencers are the major instruments or devices that help in the analyzing and processing of biological samples for the detection of genetic abnormalities with cancer.
U.S. Oncology Molecular Diagnostics Market Recent Developments
- In April 2024, Naveris, Inc., a leading provider of precision oncology diagnostics for viral-induced cancers launched Phase II clinical study in minimal residual disease positive (MRD+) HPV-driven neck and head cancer with the leading cancer research and institution Memorial Sloan Kettering Cancer Center (MSKCC).
- In July 2022, a next-generation molecular diagnostics organization, BillionToOne, launched Northstar Select and Northstar Response, its first liquid biopsy products.
- In April 2024, Envisagenics, an artificial intelligence driven biotechnology organization announced the launch of its journal Molecular Systems Biology the study is the evaluation of the organization’s SpliceCore AI/ML platform in Triple Negative Breast Cancer (TNBC).
- In April 2024, Genetic Technologies Limited, a leading global provider of genomics-based test in wellness, health, and serious disease announced the launch of company’s precision oncology division and the portfolio of the latest diagnostics tests under the gene Type precision oncology brand.
- In April 2024, Labcorp, a leading brand in the innovative and comprehensive laboratory services launched the Labcorp® Plasma Detect™, a clinically authorized tumor-informed molecular residual disease (MRD) and whole-genome sequencing circulating tumor DNA (ctDNA) solution for the early stage of colon cancer patients with the higher risk after the surgery or adjuvant chemotherapy (ACT).
- In April 2024, TwinStrand Biosciences®, a leading player in Duplex Sequencing technology that delivers the precise results to researchers from the applications from genetic toxicology to residual cancer detection announced the acceptance of standard project submission form (SPSF) from the company for Economic Cooperation and Development (OECD).
U.S. Oncology Molecular Diagnostics Market Top Key Companies:
- Roche Diagnostics
- Abbott Laboratories
- Thermo Fisher Scientific Inc.
- Illumina, Inc.
- Bio-Rad Laboratories, Inc.
- Myriad Genetics, Inc.
- Agilent Technologies, Inc.
- Danaher Corporation (Cepheid)
- Hologic, Inc.
- Foundation Medicine, Inc.
U.S. Oncology Molecular Diagnostics Market Report Segmentation
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2033. For this study, Nova one advisor, Inc. has segmented the U.S. Oncology Molecular Diagnostics market.
By Type
- Breast Cancer
- Liver Cancer
- Prostate Cancer
- Colorectal Cancer
- Cervical Cancer
- Lung Cancer
- Blood Cancer
- Kidney Cancer
- Other Cancer
By Technology
- PCR
- Sequencing
- In Situ Hybridization
- INAAT
- Chips and Microarrays
- Mass Spectrometry
- TMA
- Others
By Product
- Reagents
- Instruments
- Others

